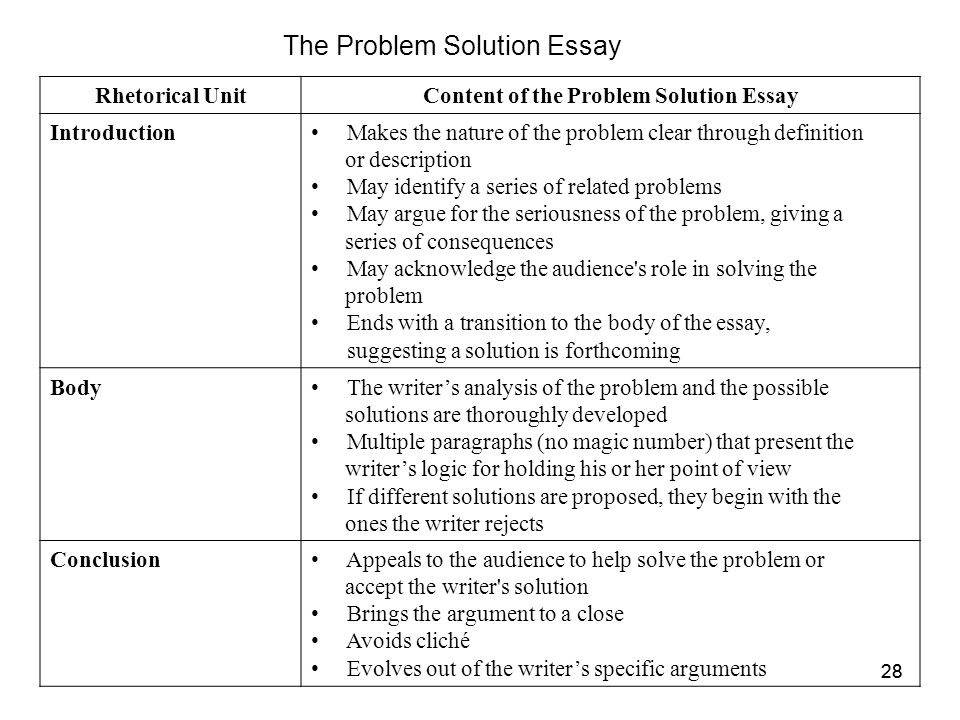
Naming and Writing Formulas for Acids!
Acids and bases are used in many chemical reactions. They are responsible for most color change reaction and are used to adjust the pH of chemical solutions. Here are the names of some of the common acids and bases and the formulas associated with them.
IMGT classes of the 20 common amino acids. Amino acid abbreviations, characteristics, volume and hydropathy index. Charge, hydrogen donor and acceptor atoms, and polarity of the amino acid side chains. Formula of the 20 common amino acids and structural details of the side chains. Formula of the 20 common amino acids.

To write the formula and names of three common strong acids and strong bases. Concept Introduction: Electrolytes are the substance whose aqueous solution conducts electricity. The strong bases have following properties: In solution, strong bases ionize fully. For strong bases, the value of equilibrium constant ( K b) is large.

Start studying Formulas for Acids. Learn vocabulary, terms, and more with flashcards, games, and other study tools.

Answers: Acids Write the formula for each of the acids listed below: Nitric acid HNO 3: Hydrocyanic acid HCN (aq) Chloric acid HClO 3: Acetic acid CH.

Naming and Writing Formulas for acids and bases by linda. . Chemistry.

Amino acids. An amino acid contains both an amino group, -NH 2, and a carboxylic acid group, -COOH, in the same molecule. As with all acids the carbon chain is numbered so that the carbon in the -COOH group is counted as number 1. Example: Write the structural formula for 2-aminopropanoic acid.
Recall the general formula for saturated fatty acids List some examples of saturated fatty acids and foods they are found in To unlock this lesson you must be a Study.com Member.

Write the formula for each of the following acids. hydrocyanic acid nitric acid sulfuric acid phosphoric acid hypochlorous acid hydrobromic acid bromous acid hydrofluoric acid.

Chemical formula of acid. There are many different types of acids. Acids taste sour, turn blue litmus paper pink and have a pH of less 7. Some acids are very corrosive like sulfuric acid, nitric acid and hydrochloric acid. These acids can cause damage to the skin, eyes and clothing. They also vigorously react with active metals such as magnesium, zinc and iron producing hydrogen gas and salts.

Answer to Write the formula for the conjugate base of each of the following acids. HNO2 H2SO3 HCHO2 HClO.

How to Write Formulas for Acids. Compounds beginning with H (Hydrogen) are usually considered to be acids. When were asked to write the formula for an acid, when given the name, we follow a set of rules depending on the type of acids.

How do you write a chemical formula for acids? Wiki User 2010-11-16 00:48:45. This is a very undefined question as there are an almost. infinite number of reactants and resulting products. All.